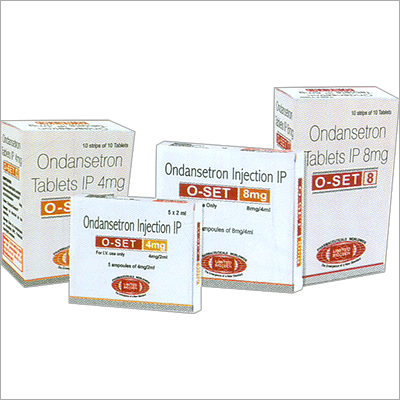
Ondansetron Injection IP
10 INR
Product Details:
- Enzyme Types Emulsifiers
- Feature high Fermentation Activity
- Physical Color/Texture Tan liquid
- Fermentation Smell Normal Smell
- Storage Instructions Dry Place
- Click to View more
X
Ondansetron Injection IP Price And Quantity
- 3000 Piece
- 10 INR
Ondansetron Injection IP Product Specifications
- high Fermentation Activity
- Tan liquid
- Normal Smell
- Emulsifiers
- Dry Place
Ondansetron Injection IP Trade Information
- Telegraphic Transfer (T/T)
- 3000 Piece Per Day
- 1 Week
- Yes
- Free samples are available
- Western Europe Australia Eastern Europe Middle East Central America Africa South America Asia North America
- All India
Product Description
Ondansetron Injection IP, Ondansetron Tablets IP 8mg
| Product | Adult dose | Pediatric dose | Adjustment in Renal / Hepatic Impaired patients |
| O-Set | Inj: up to 32mg/day in divided doses Tab:24mg, 30min before start of chemotherapy | 150 mcg/kg of body weight | No |
| Product name | Composition | Indication/s | Strengths | Packing |
| O-Set | Ondansetron | Prevention of cancer chemotherapy-induced nausea and vomitting, prevention of nausea and vomiting associated with radiotherapy | 4mg | 1 amp |
| 8mg | 1 amp | |||
| 4mg | 1x10 Tabs | |||
| 8mg | 1x10 Tabs |
| Product name | Storage | Stability after reconstitution | Prep of solution | Diluent |
| O-Set | Protect from light. Store below 25oC | Unpreserved ondansetron injection has been shown to be stable for 7 days at room temperature (below 25oC) or in a refrigerator | Intravenous solutions should be prepared at the time of infusion. Should be diluted in 50ml of 5% dextrose injection or 0.9% NaCI injection infuse for 15 minutes | 0.9% NaCI solution, 5% Dextrose injection. |
Other Products in 'Oncology Drugs' category
|
|
|
 |
UNITED BIOTECH (P) LTD.
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |